Le Chatelier

8 october 1850 17 september 1936 was a french chemist of the late 19th and early 20th centuries.
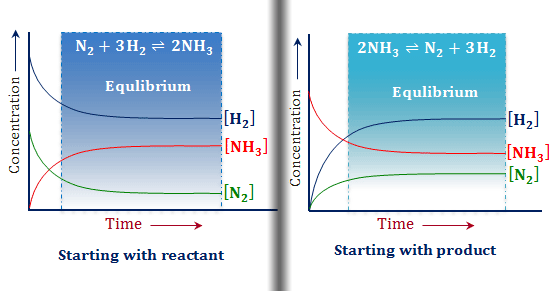
Le chatelier. El principio de le chatelier predice que el sistema se ajustará para huir del efecto causado por la remoción de h2. Le chatelier s principle pronounced uk. Parte del hi se descompone para formar h2 para sustituir lo que fue retirado. Henry louis le chatelier french pronunciation.
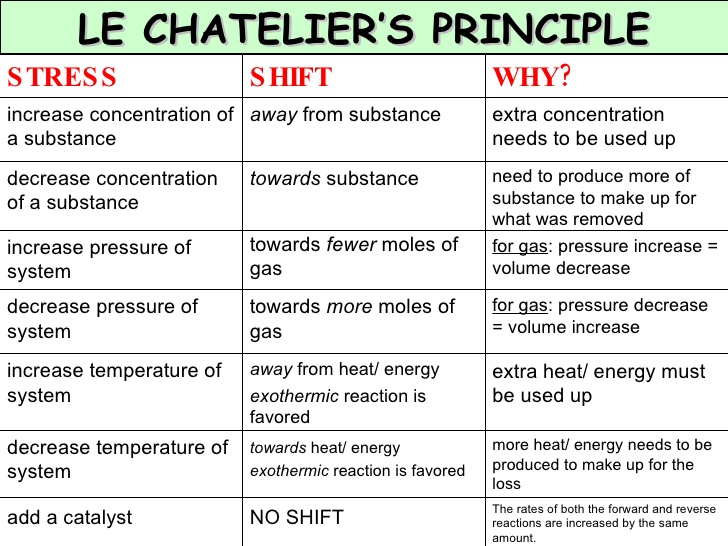
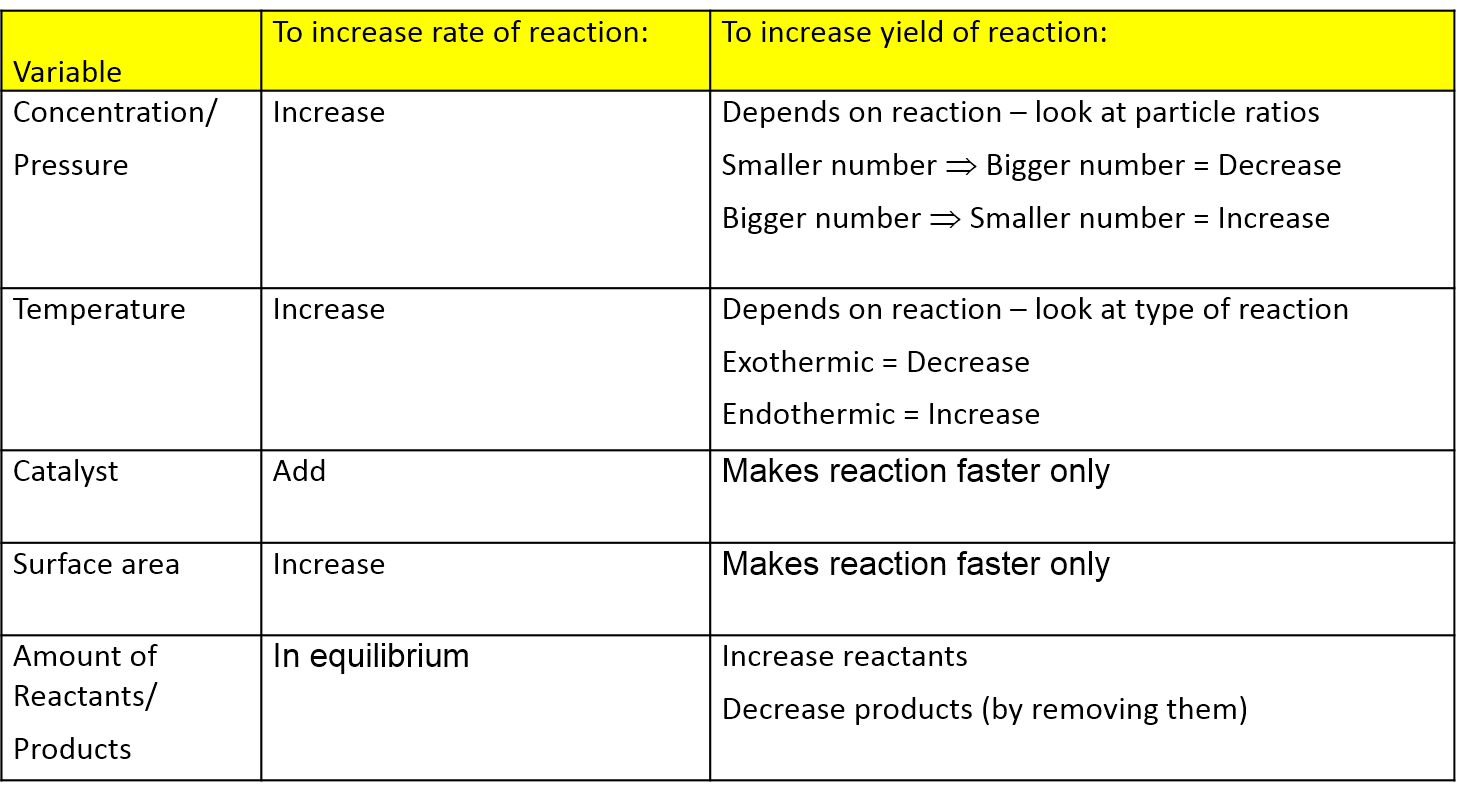
Le chatelier s principle is the principle when a stress is applied to a chemical system at equilibrium the equilibrium will shift to relieve the stress. That means that the position of equilibrium will move so that the temperature is reduced again. 8 1850 paris france died sept. 17 1936 miribel les échelles french chemist who is best known for le chatelier s principle which makes it possible to predict the effect a change of conditions such as temperature pressure or concentration of reaction components will have on a chemical reaction.
In other words it can be used to predict the direction of a chemical reactionin response to a change in conditions of temperature concentration volume or pressure. Suppose the system is in equilibrium at 300 c and you increase the temperature to 500 c. It states that changes in the temperature pressure volume or concentration of a system will result in predictable and opposing changes in the system in order to achieve a new equilibrium state. El efecto obtenido es la disminución de la concentración del hi y al aumento de la concentración del i2.
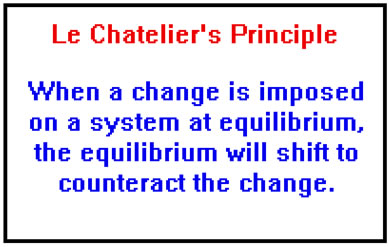
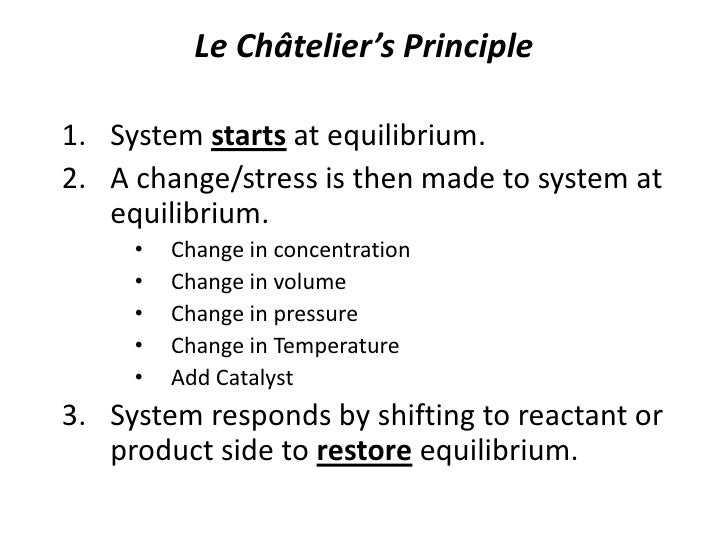
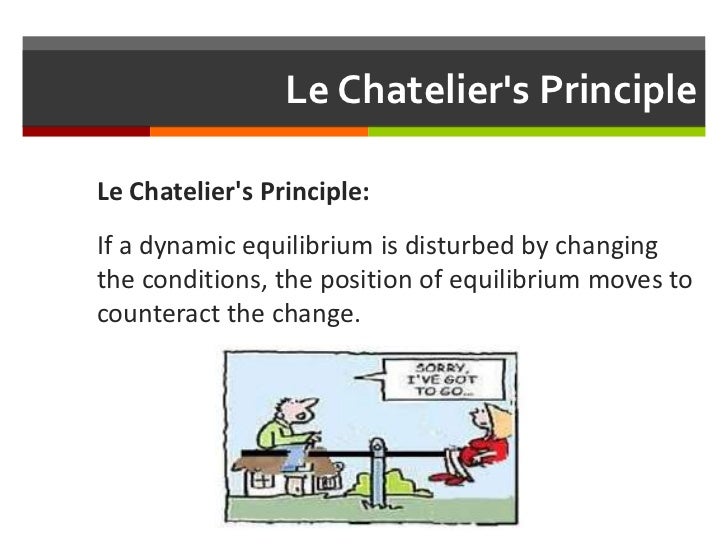
According to le chatelier the position of equilibrium will move in such a way as to counteract the change. Le chatelier s principle is an observation about chemical equilibria of reactions. To restore equilibrium the reaction will in either forward or backward direction. ˈ ʃ ɑː t əl j eɪ also called chatelier s principle or the equilibrium law is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria.
He devised le chatelier s principle used by chemists and chemical engineers to predict the effect a changing condition has on a system in chemical equilibrium. According to le chatelier s principle a change in temperature is a stress on an equilibrium system. Le chatelier s principle states that if a dynamic equilibrium is disturbed by changing the conditions such as concentration temperature and pressure changes the position of equilibrium shifts to counteract the change to reestablish an equilibrium. Henry louis le chatelier born oct.