Le Chatelier S Principle Pressure And Temperature
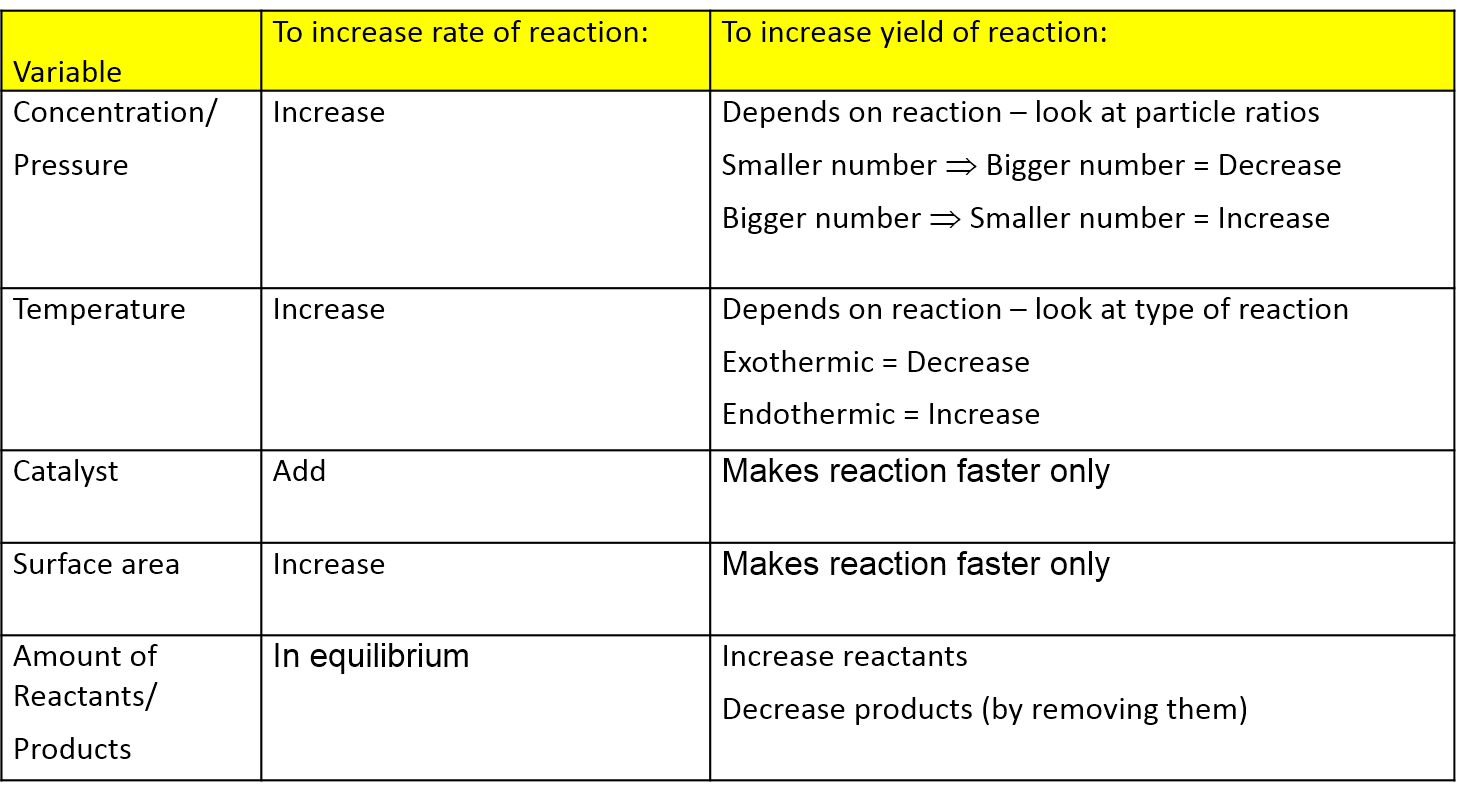
Le chatelier s principle implies that the addition of heat to a reaction will favor the endothermic direction of a reaction as this reduces the amount of heat produced in the system.
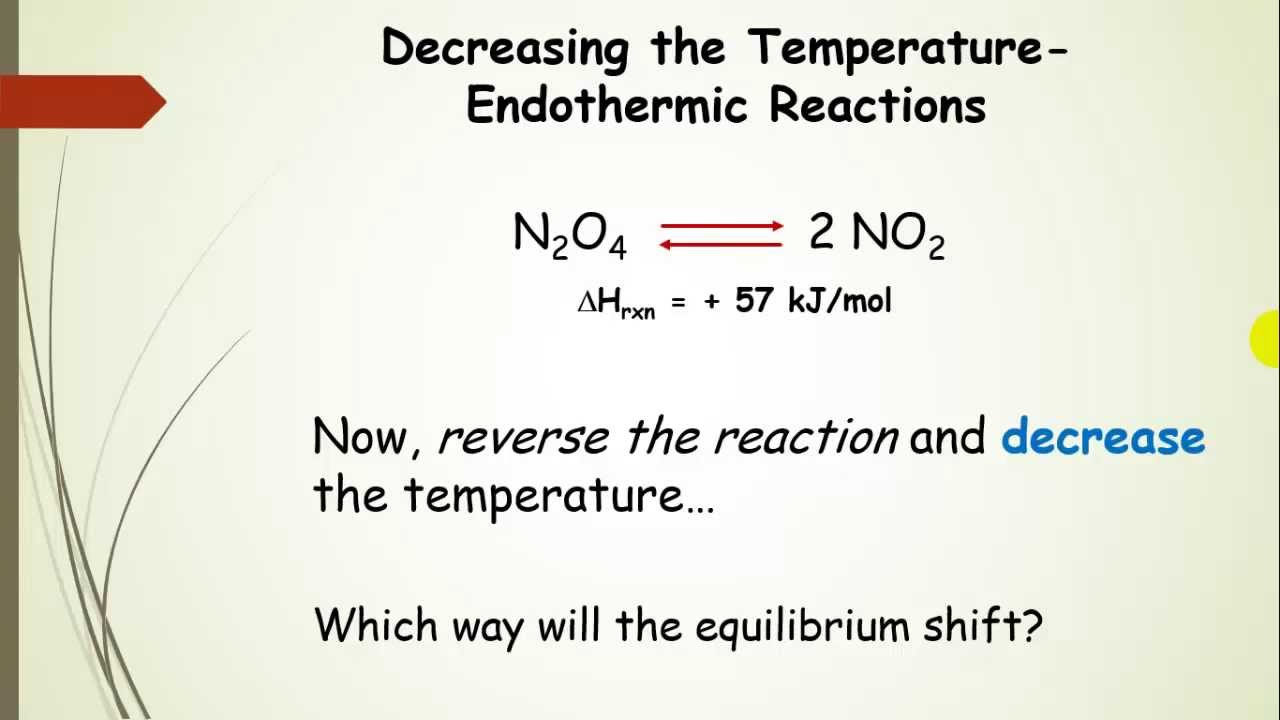
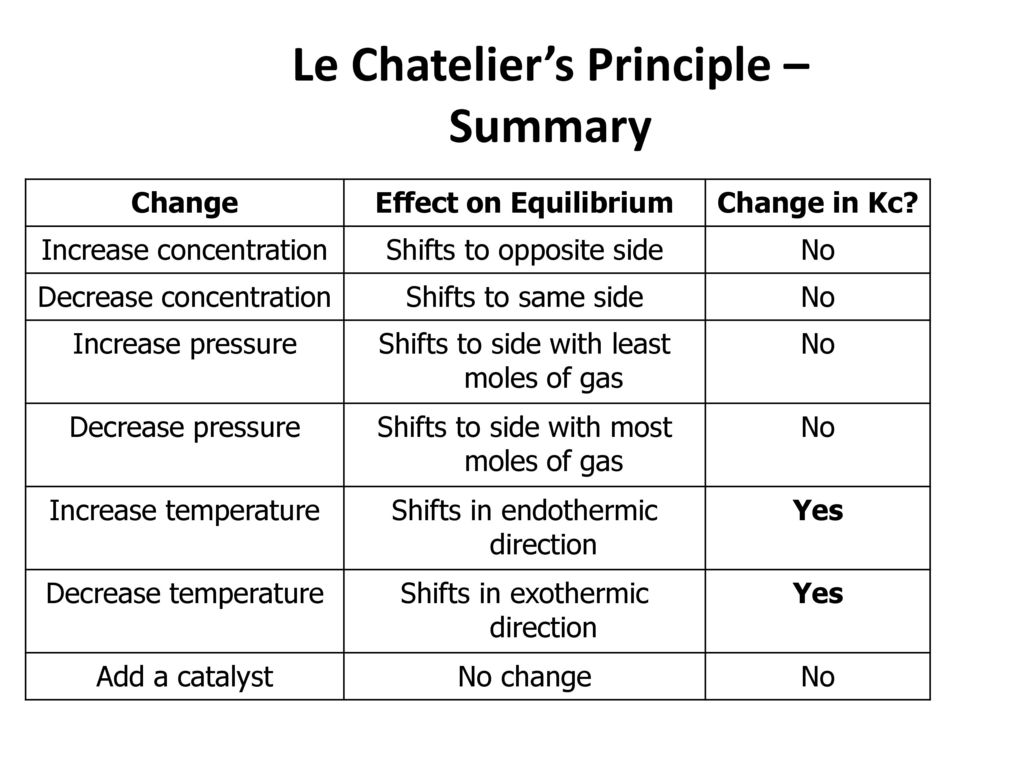
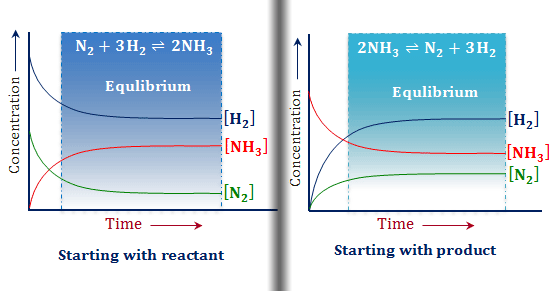
Le chatelier s principle pressure and temperature. It covers changes to the position of equilibrium if you change concentration pressure or temperature. According to le chatelier s principle a change in temperature is a stress on an equilibrium system. That means according to le chatelier s principle the synthesis of ammonia is favored at lower temperatures. Le chatelier s principle is the principle when a stress is applied to a chemical system at equilibrium the equilibrium will shift to relieve the stress.
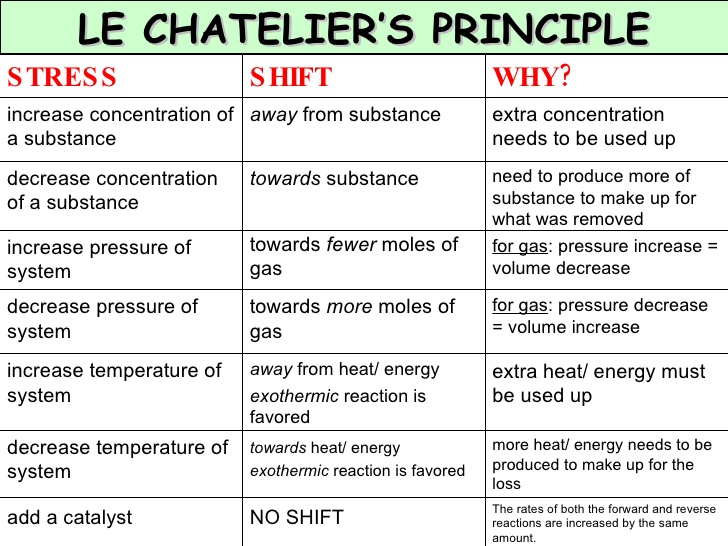
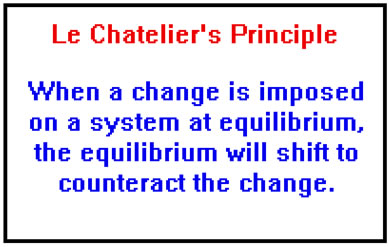
Le chatelier s principle a c to this principle if a system is subjected to a change of concentration pressure or temperature then the equilibrium shifts in the direction that tends to undo the effect of this change. It also explains very briefly why catalysts have no effect on the position of equilibrium. To restore equilibrium the reaction will in either forward or backward direction. Le chatelier s principle also called chatelier s principle or the equilibrium law is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria.
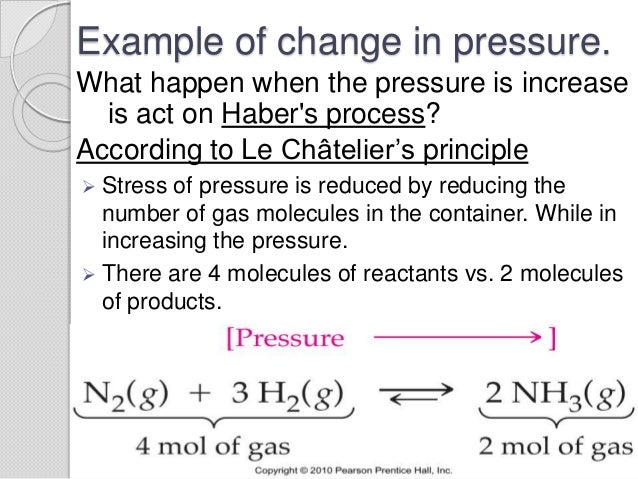
If at equilibrium the temperature of system is changed the system will no longer at remain at equilibrium. When any system at equilibrium for a long period of time is subjected to a change in concentration temperature volume or pressure. Industrially 100 250 atm. This page looks at le chatelier s principle and explains how to apply it to reactions in a state of dynamic equilibrium.
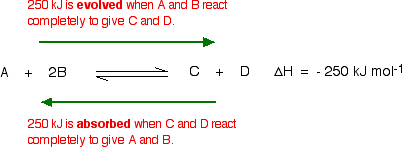
It can be stated as. Since the forward reaction is exothermic the increase in temperature favors the backward reaction i e the dissociation of ammonia. Le chatelier s principle in a system under equilibrium if there is change in pressure temperature or concentration then the equilibrium shifts in such a manner as to reduce or counteract the reflect of change. The principle is named after french chemist henry louis le chatelier and sometimes also credited to karl ferdinand braun who discovered it independently.
Le chatelier s principle can be used to predict the behavior of a system due to changes in pressure temperature or concentration. Of pressure is employed.






.jpg)